Official Submissions
Official Submission Process
The folowing steps must be met for your product submission
1. A cover letter that includes the name of the specific product(s) and the specific claim(s) [plaque and/or tartar] requested for VOHC review. The cover letter is to be signed by the responsible official of the submitting company, and is to indicate to whom VOHC correspondence regarding the submission is to be sent.
2. Pay the submission fee
3. An affidavit signed by the primary investigator and by the responsible official of the sponsoring company, stating that the information submitted is complete and true, and including the following statements, with signature validated by a Notary Public or equivalent.
Sample Affidavit Language
4. Trial Protocol Form-This would be the Form with the additions and corrections from the VOHC from your Pre-Trial Protocol Form found in step one.
5. A summary table showing the mean mouth scores of each group and % difference between groups (defined as: [Control minus Test] divided by Control x 100), plus a paragraph or table describing the results of the statistical analysis. The individual tooth scores and mean mouth scores for each animal are to be included in electronic spreadsheet format. Include also the identification number or name, body weight (at Day -1 or Day zero), age and sex of the individual animals. Separate tables are required for Plaque and Calculus.
6. Include a table of Gingivitis score results, including % difference between test and control groups.
7. A statement that the trial was completed as per the protocol, or a description of any out-of-protocol events.
8. Indication of any areas in which the submission does not meet specific VOHC requirements, with reasons why VOHC should consider such departures from its stated policies or requirements. This could be anything from a notation that one animal was missing one of the recommended teeth, to use of an entirely new method of scoring plaque or tartar.
9. Samples of the product (25 samples of individual treats, gels, dentifrices or brushes/devices, or one bulk package of a dietary product or one bottle of a water additive) are to be shipped to the VOHC office when the submission is sent for review; include also one sample of a placebo product used in trials of a gel or dentifrice. If the submission is on behalf of a piece of equipment that would be expensive to send as samples, contact the VOHC office – a video of the equipment in use may be sufficient.
Submission Fee Policy:
1. A Submission Fee (see Fee Table below) is to be paid at the time of submission of a product for review by VOHC.
2. The Submission Fee is not refunded if the submission is reviewed and product is not awarded the VOHC Seal.
3. An Annual Maintenance Fee (see Fee table) will be billed on the anniversary of the awarding of the Seal.
4. The VOHC Accepted® Seal is awarded initially for a 3-year period. The Agreement will be extended by VOHC provided the Product has not been changed in any significant way and provided the VOHC Efficacy Standard has not changed such that the data included in the Submission show that the product no longer meets the VOHC standard.
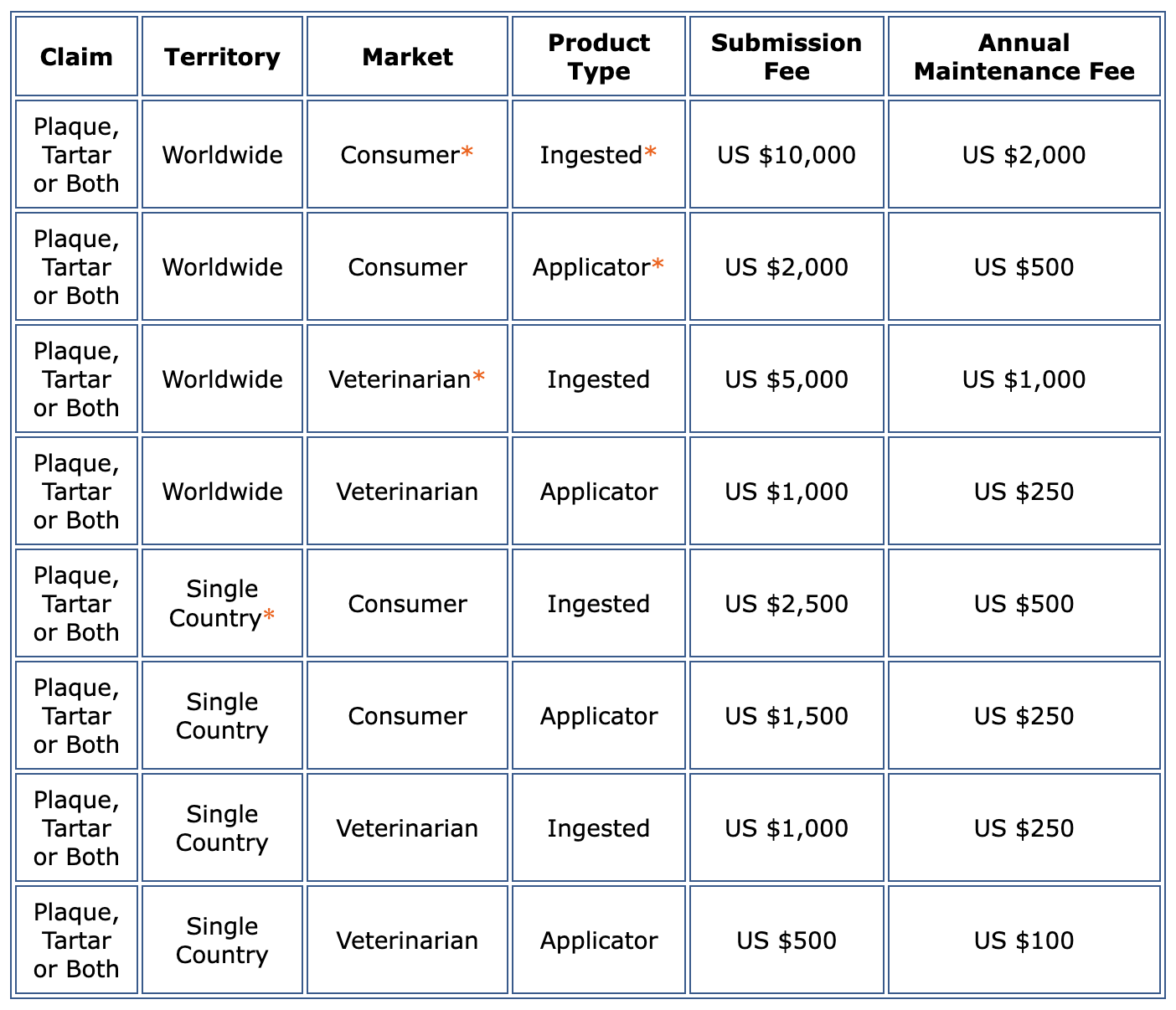
Consumer = Primarily marketed through retail stores (e.g. pet stores, supermarkets).
* Veterinarian = Marketed exclusively through veterinary clinics and hospitals.
* Ingested = Swallowed or voluntarily chewed product (e.g. diet, chew, treat, dentifrice)
* Applicator = Product used by the owner, applied to produce a mechanical action on the
surfaces of the teeth (e.g. toothbrush, dental wipe)
* The ‘single country’ fee is not applicable in North America.
For more information on these definitions, contact the VOHC Office.
